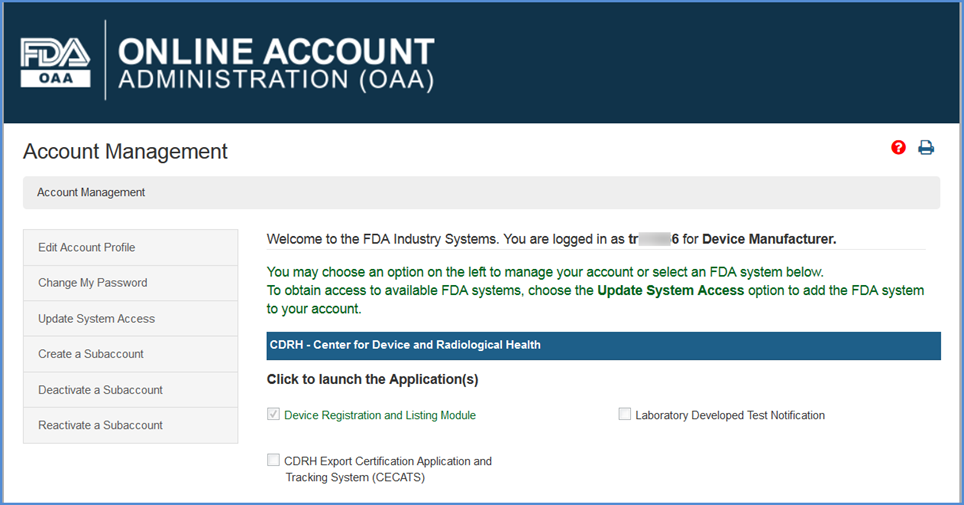
Vague FDA policies on adverse event data are keeping patients from accessing investigational drugs | Fierce Healthcare

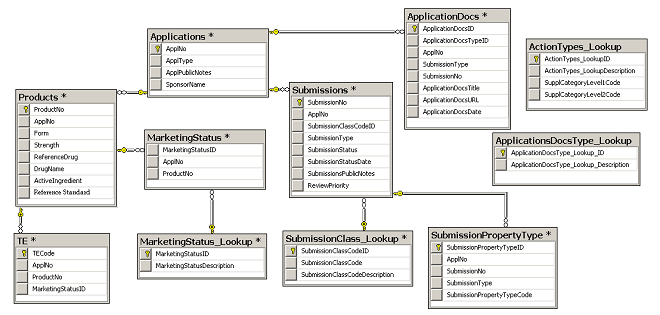
OpenFDA API, with Excel: | Harish's Notebook - My notes... Lean, Cybernetics, Quality & Data Science.
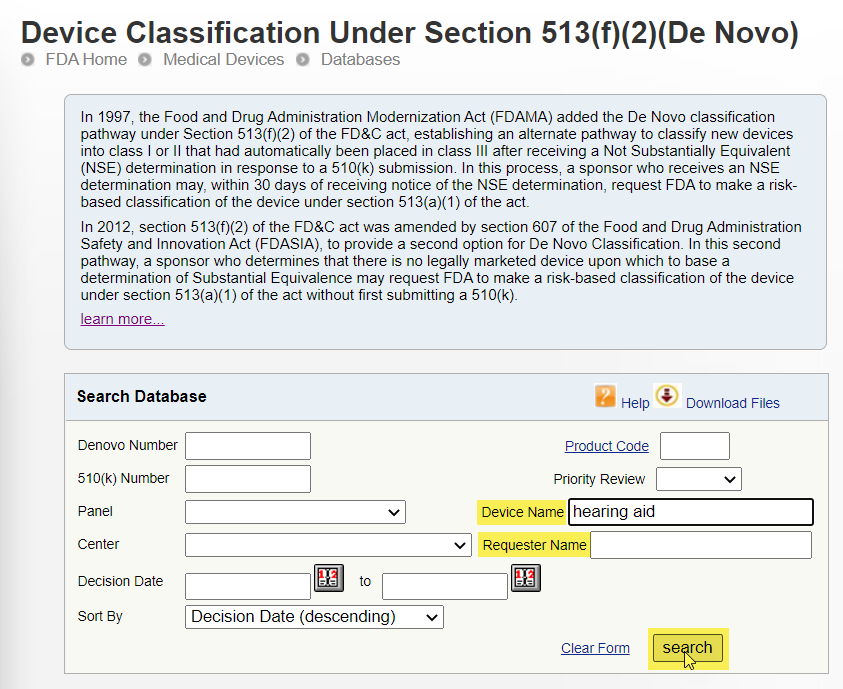
Are There "FDA Registered" or "FDA Certified" Medical Devices? How Do I Know What Is FDA Approved? | FDA

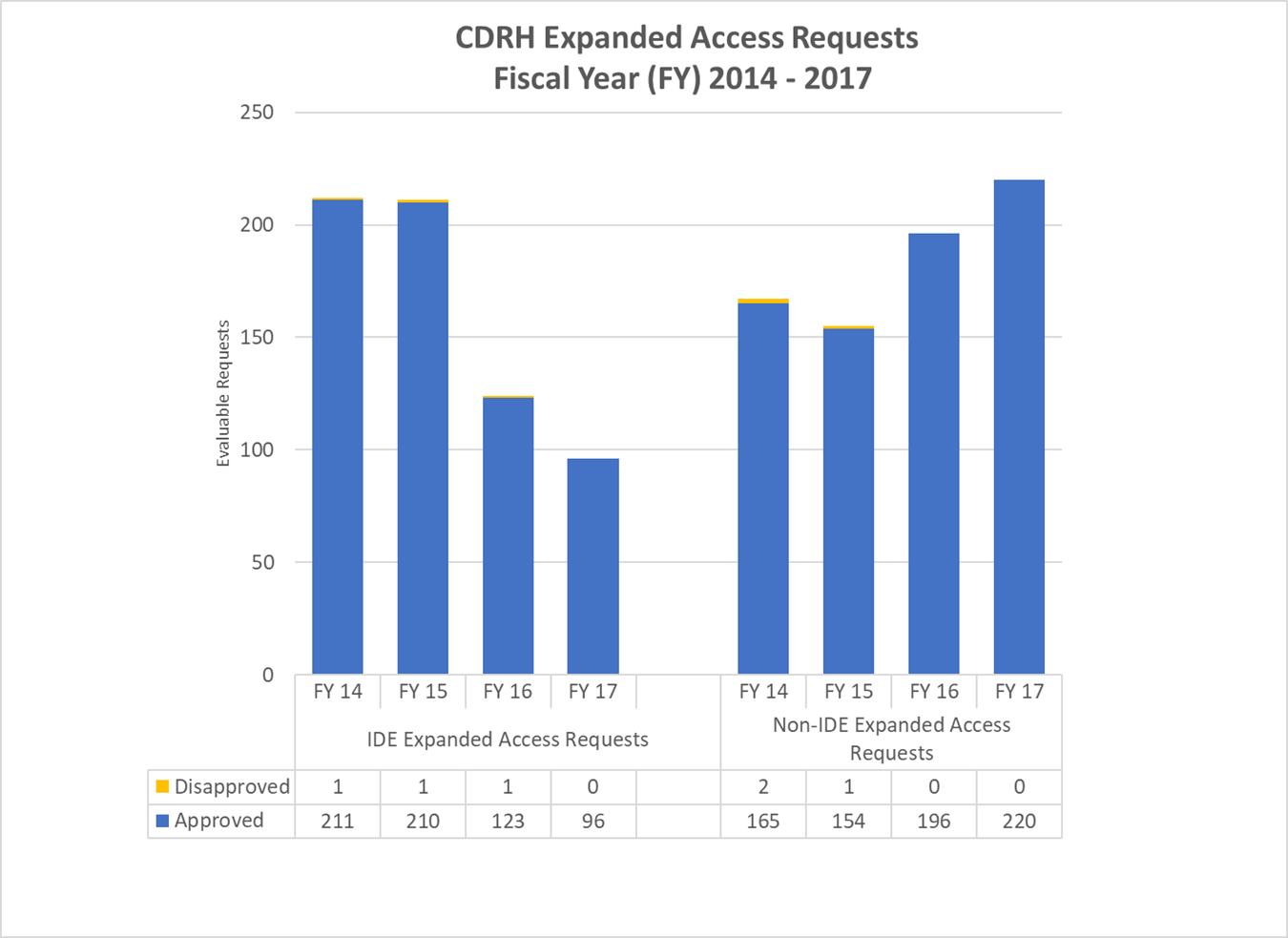
Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO

Expanded Access as a source of real‐world data: An overview of FDA and EMA approvals - Polak - 2020 - British Journal of Clinical Pharmacology - Wiley Online Library
![How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2020/05/ELM-F001-06-How-to-Avoid-FDA-Data-Integrity-System-Access-Warning-Letters-21-CFR-Part-11.png)
How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses

ClinGen: First FDA- Approved Genomic Variants Database | Innovation Center for Biomedical Informatics | Georgetown University

Navigating FDA Interactions for Early-Stage Biotechs: A Guide to Successful Regulatory Engagements | OHSU