FDA ACADEMY FREE ONLINE SEMINARS ARE NOW AVAILABLE AT THE DOH E-LEARNING PLATFORM! - Food and Drug Administration

Regulatory Best Practices for Global Access to Medicines, Including Anti-TB Medicines - Day 1 - YouTube
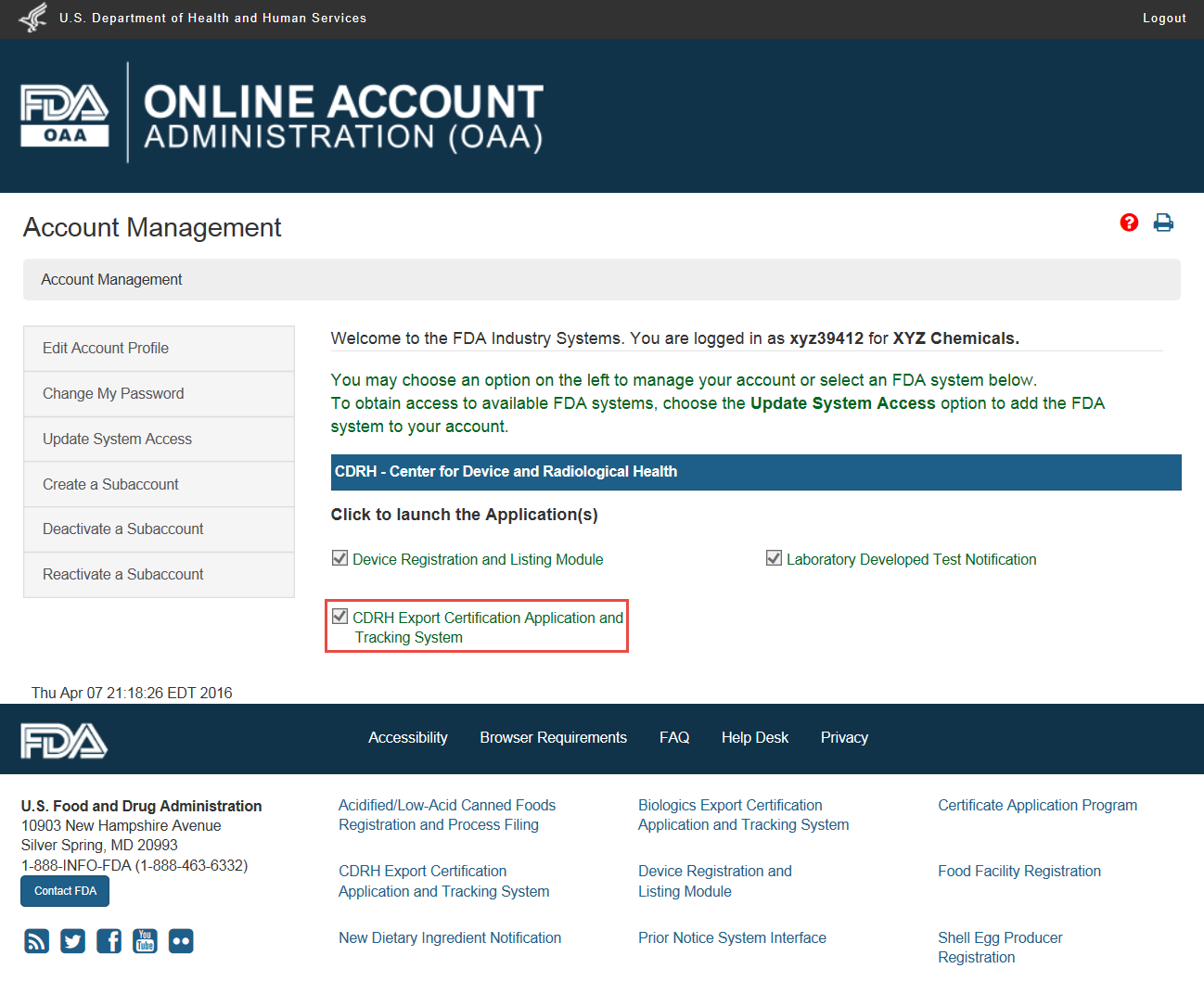
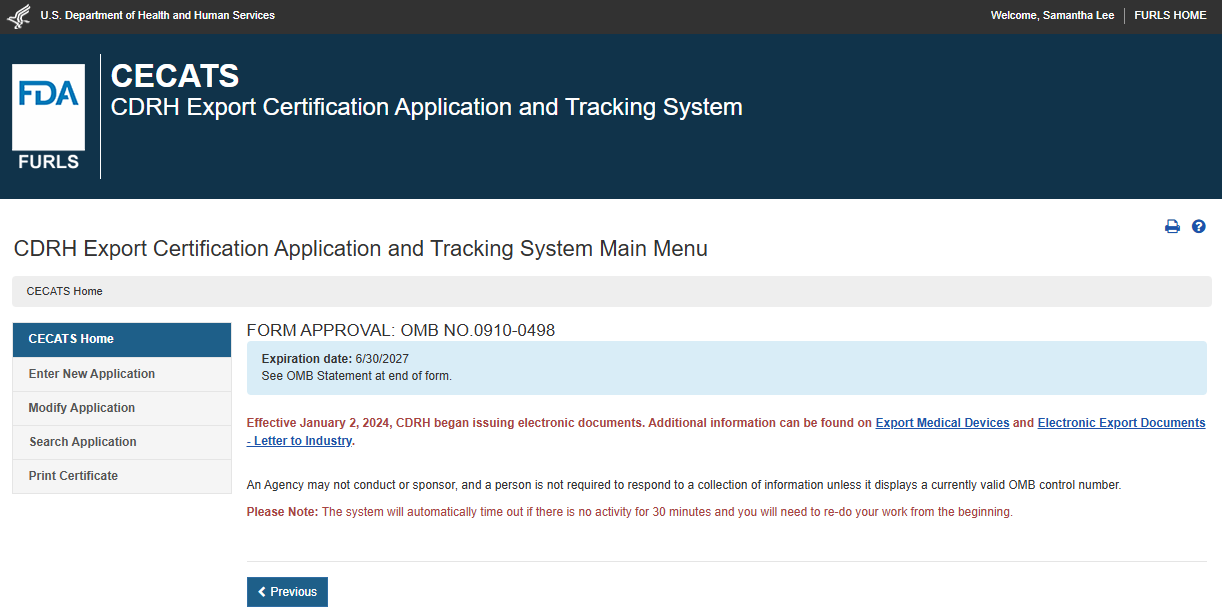
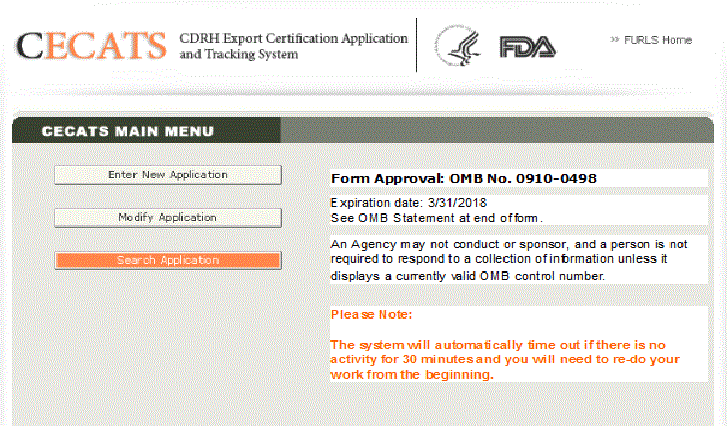
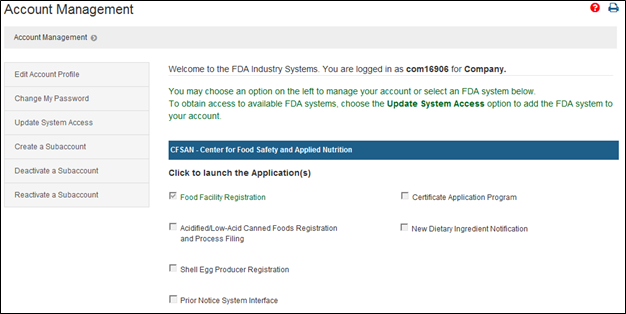
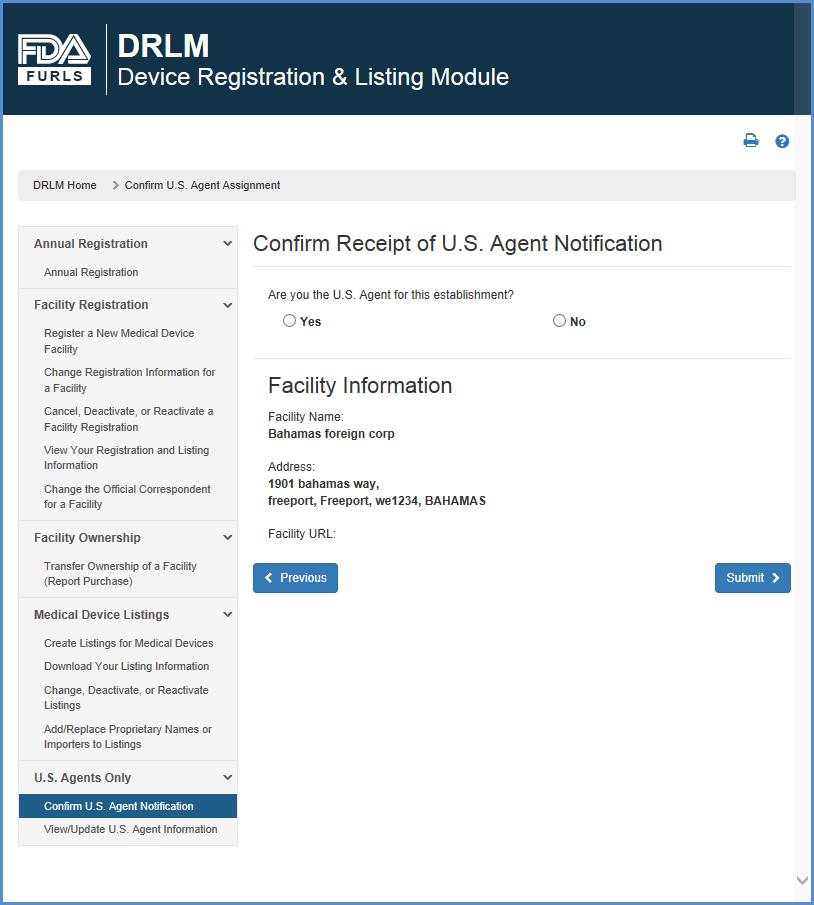
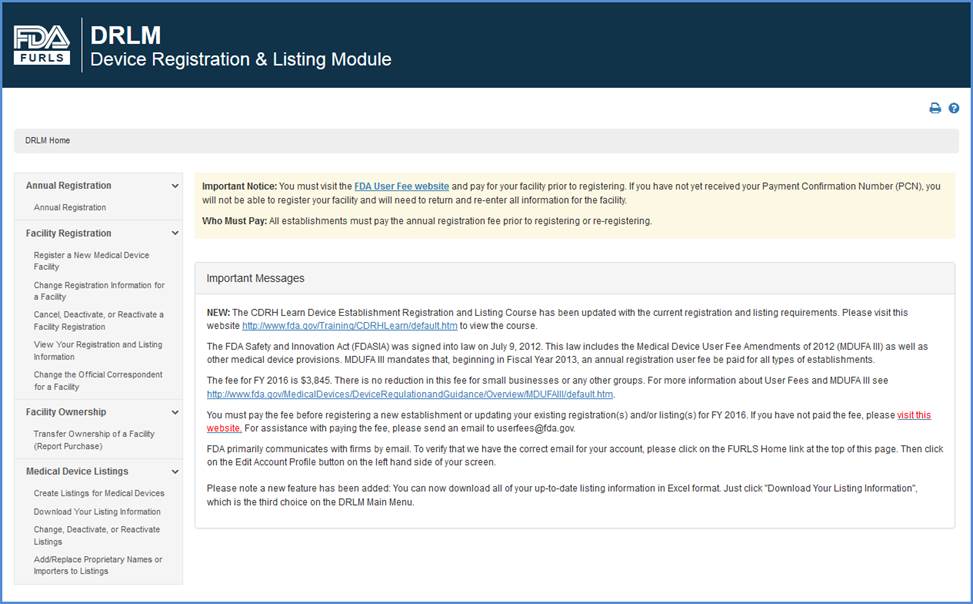
Create a Medical Device Certificate for Device Not Exported from the United States (CDNE) Application