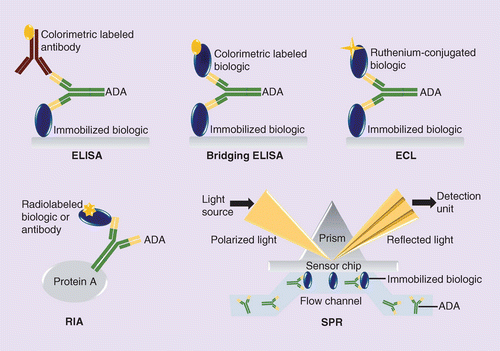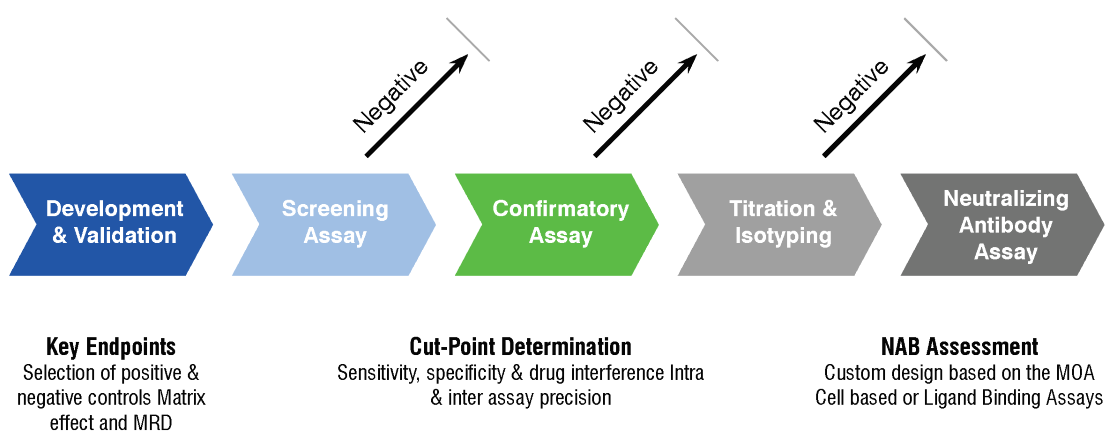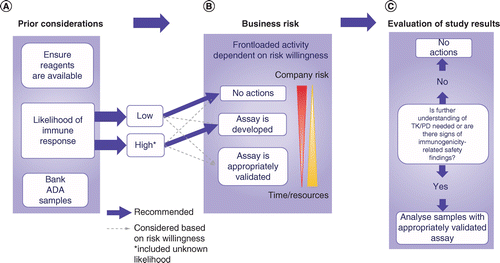
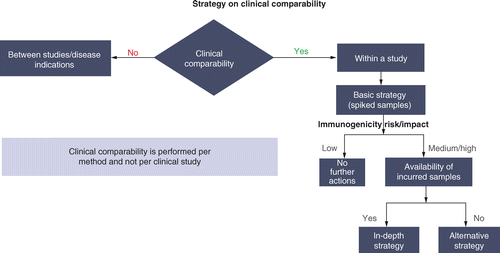
A strategic approach to nonclinical immunogenicity assessment: a recommendation from the European Bioanalysis Forum | Bioanalysis
2022 White Paper on Recent Issues in Bioanalysis: FDA Draft Guidance on Immunogenicity Information in Prescription Drug Labeling, LNP & Viral Vectors Therapeutics/Vaccines Immunogenicity, Prolongation Effect, ADA Affinity, Risk-based Approaches, NGS, qPCR,

Implementation of a three-tiered approach to identify and characterize anti-drug antibodies raised against HIV-specific broadly neutralizing antibodies - ScienceDirect

A summary comparing US FDA versus European Medicines Agency guidance on... | Download Scientific Diagram
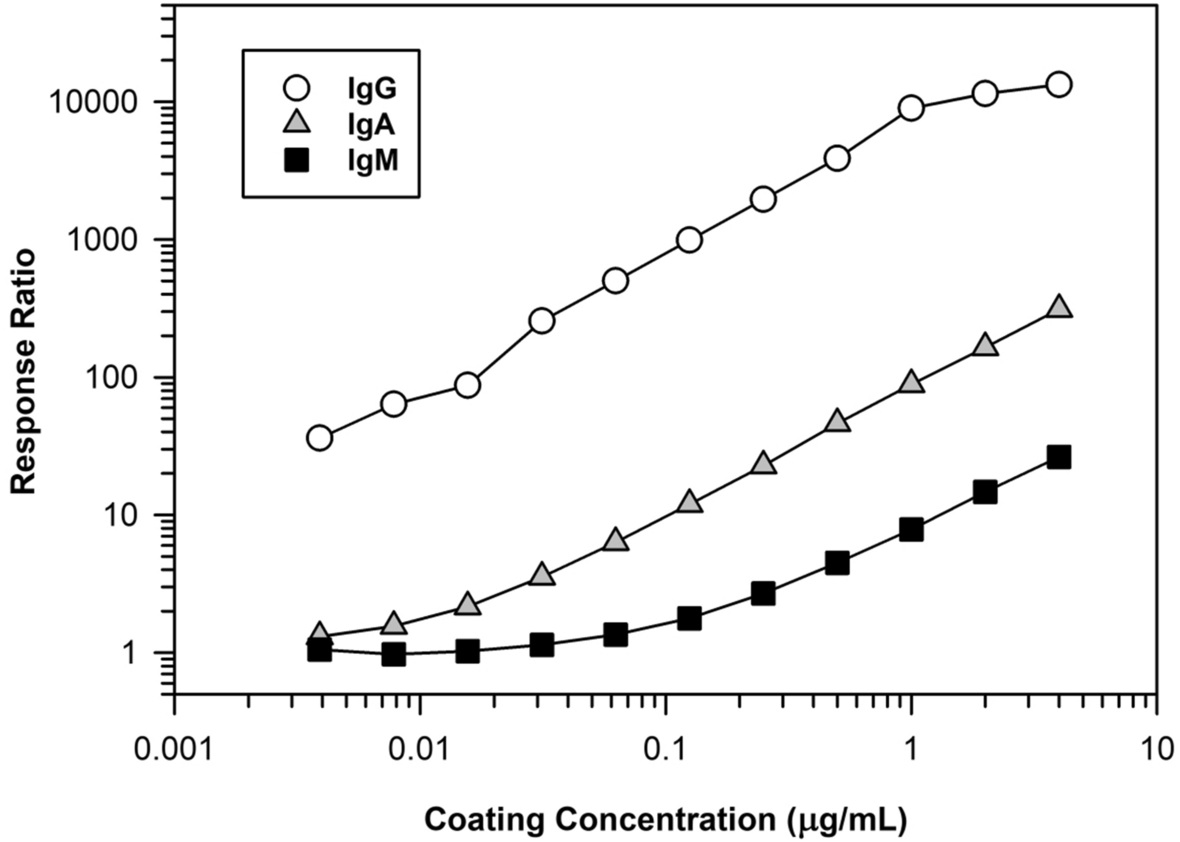
Sensitive assay design for detection of anti-drug antibodies to biotherapeutics that lack an immunoglobulin Fc domain | Scientific Reports