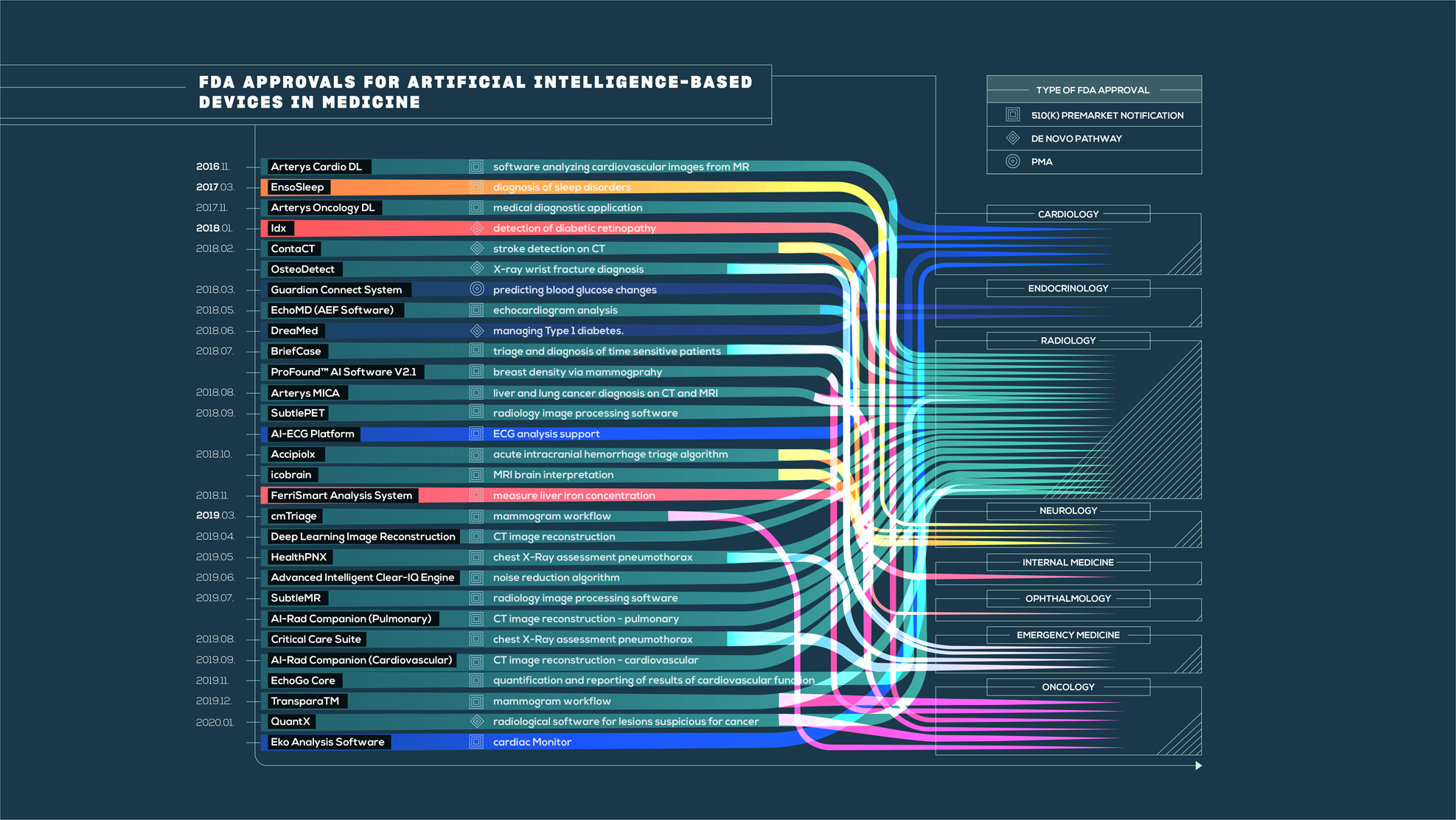
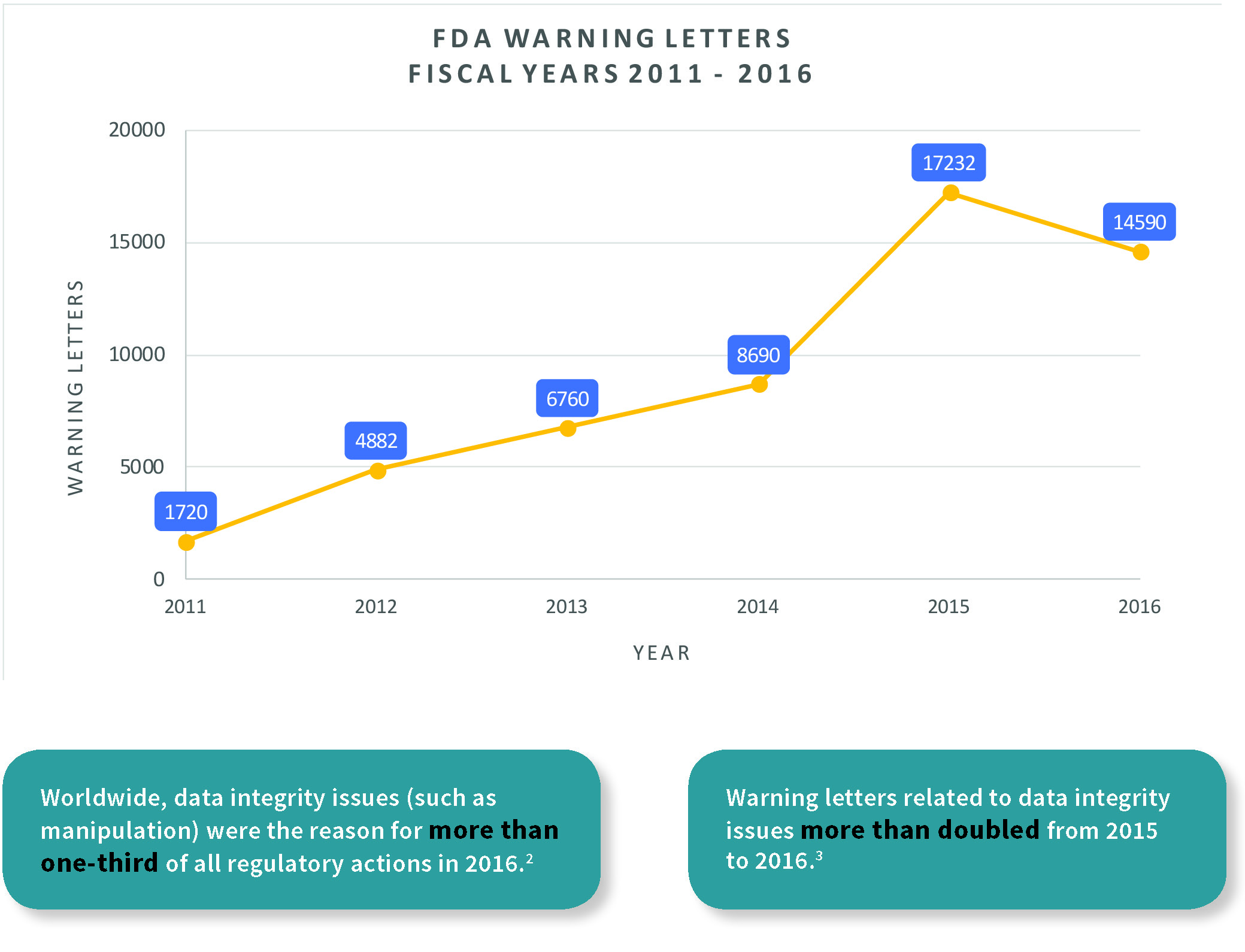
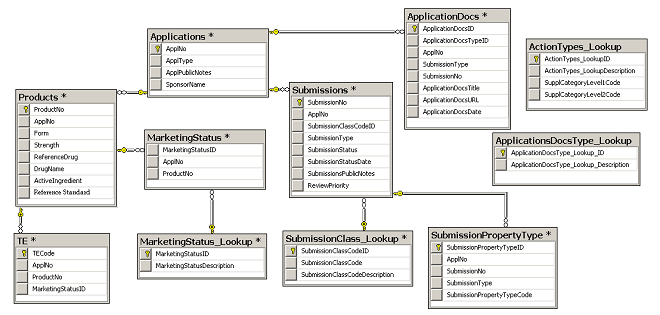
Retrieval of regulatory approval documents via the Drugs@FDA database.... | Download Scientific Diagram

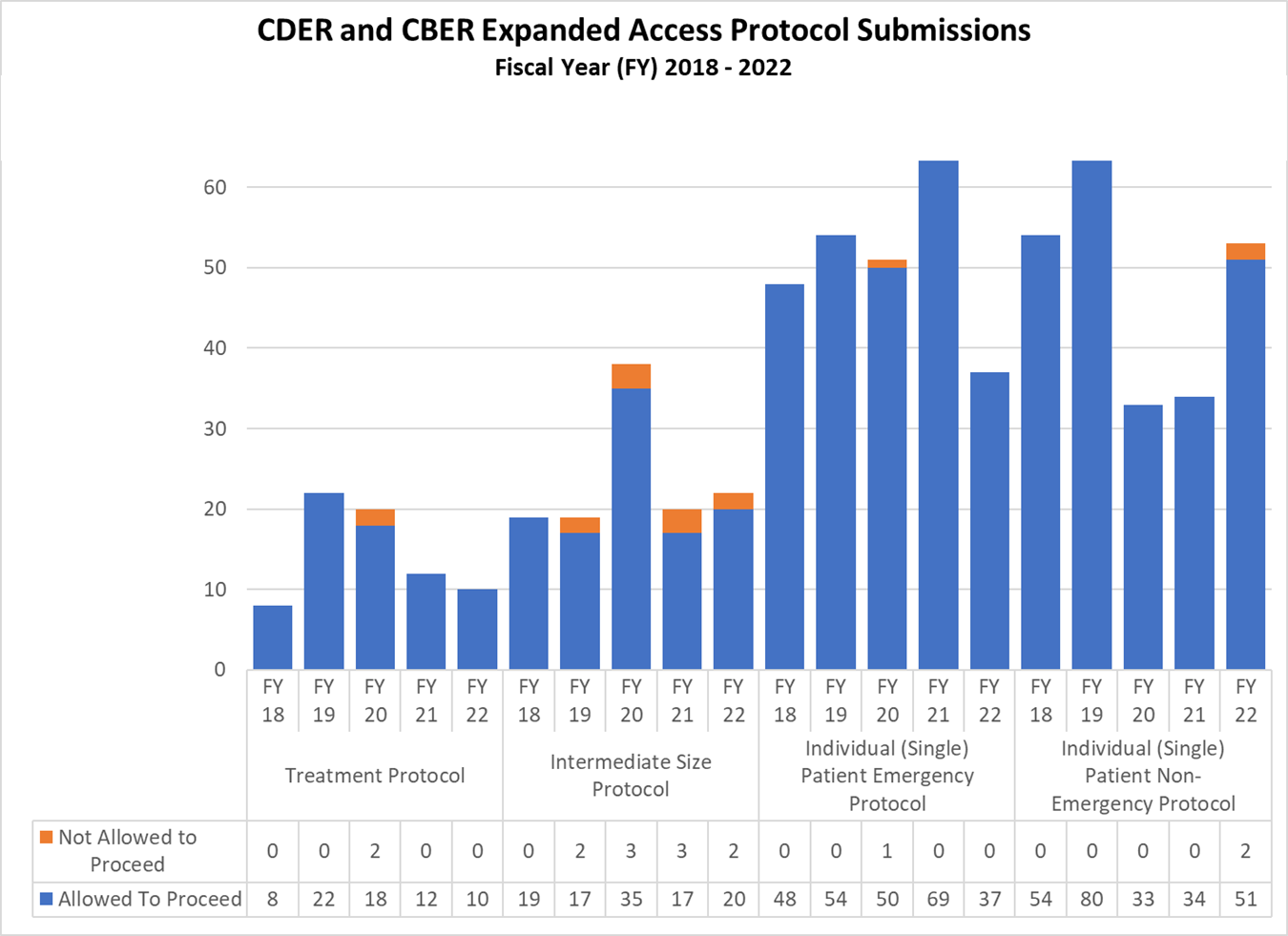
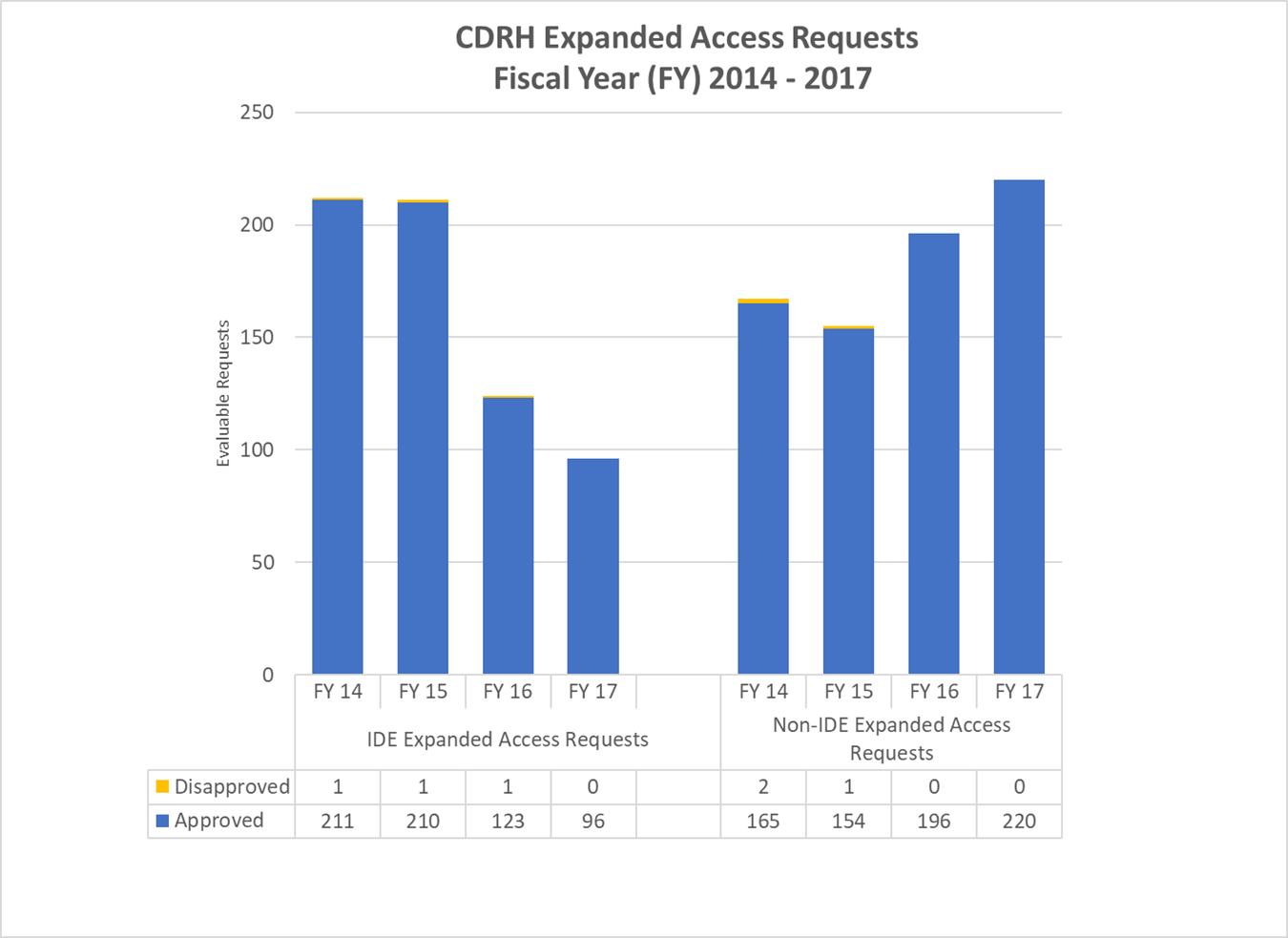
Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO

Transparency advocates win victory for public access to clinical trial data | Center for Science in the Public Interest