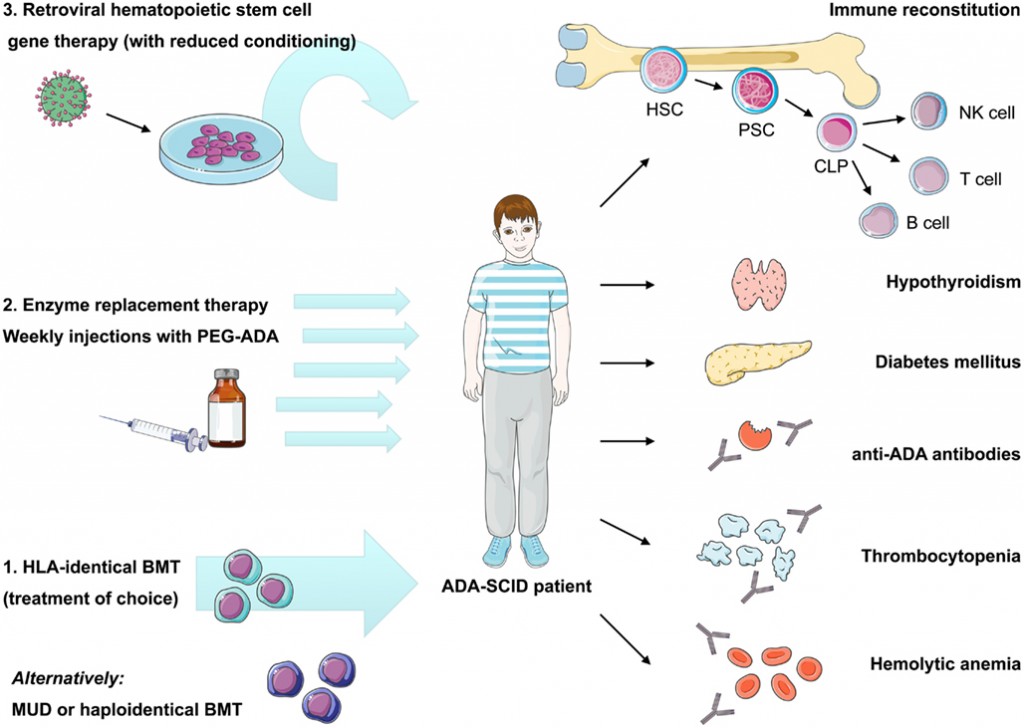
Gene therapy for ADA‐SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products | EMBO Molecular Medicine
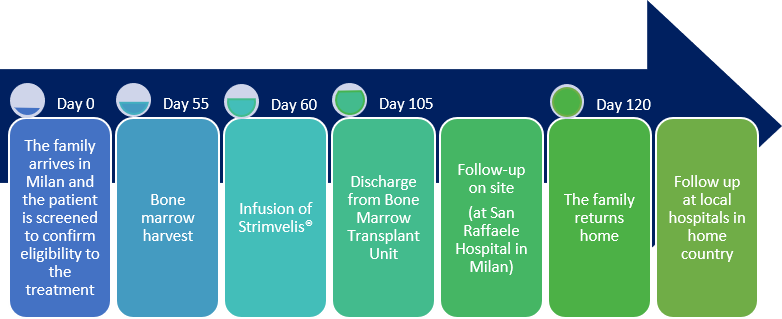
PDF) Gene therapy in rare diseases: The benefits and challenges of developing a patient-centric registry for Strimvelis in ADA-SCID

Fondazione Telethon and Orchard Therapeutics complete transfer of marketing authorization of Strimvelis for ADA-SCID in Europe - Telethon
Orchard puts dosing of Strimvelis on hold due to leukemia diagnosis in patient | S&P Global Market Intelligence

Long-term and real-world safety and efficacy of retroviral gene therapy for adenosine deaminase deficiency | Nature Medicine
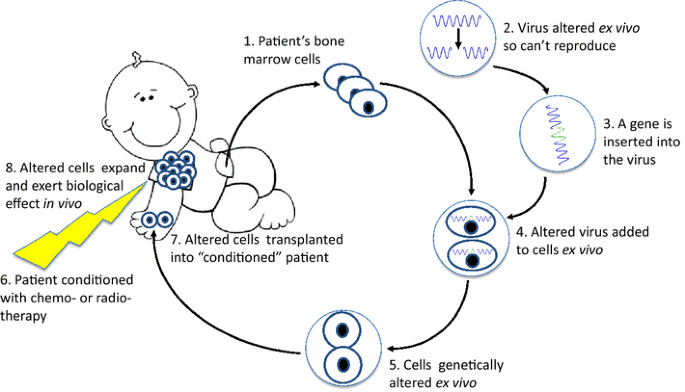
Gene therapy for primary immune deficiencies: a Canadian perspective | Allergy, Asthma & Clinical Immunology | Full Text
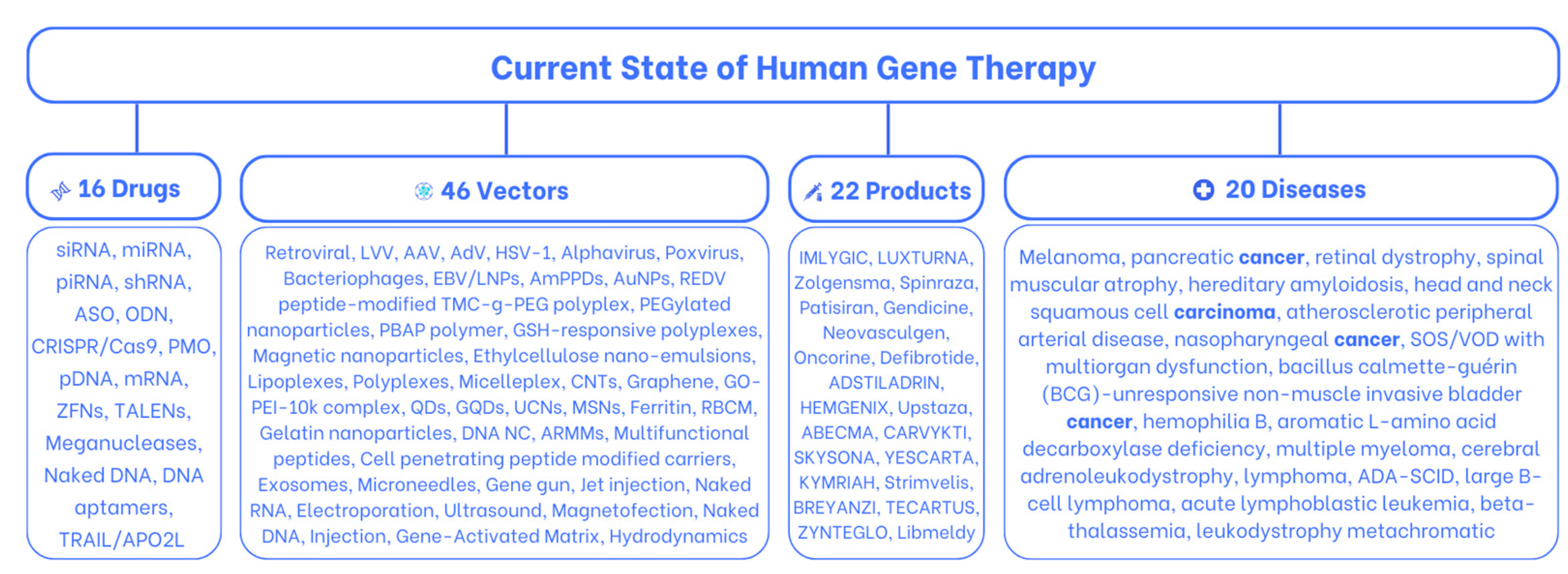
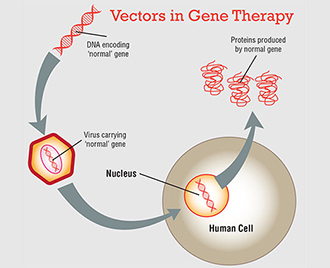
Pharmaceuticals | Free Full-Text | Current State of Human Gene Therapy: Approved Products and Vectors

Andy Biotech on X: "$GSK receives positive CHMP opinion for #GeneTherapy for 'bubble boy' disease(ADA-SCID) https://t.co/xmPWoaR3hA https://t.co/aTRCTInQQ0" / X