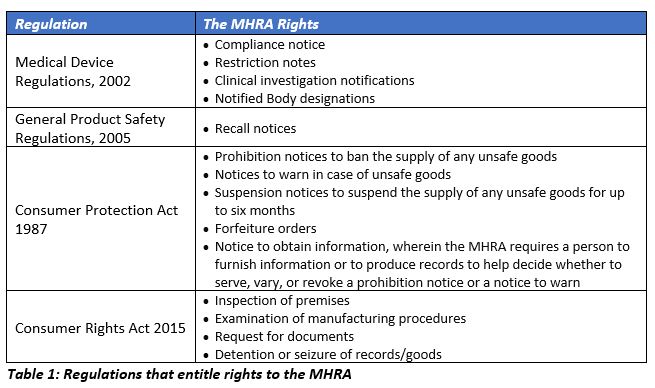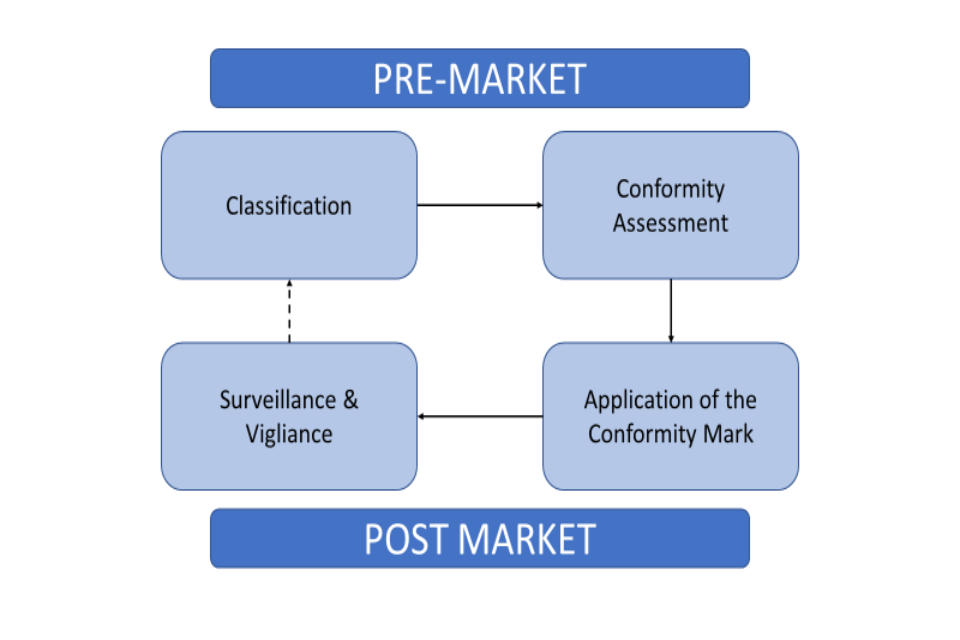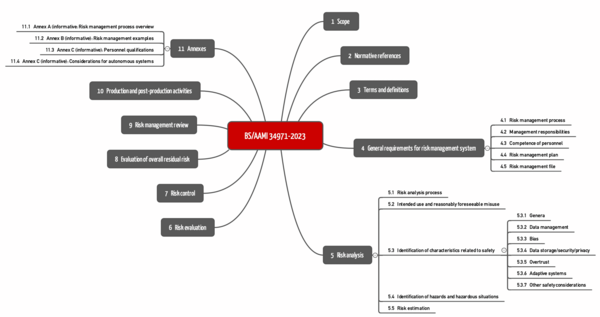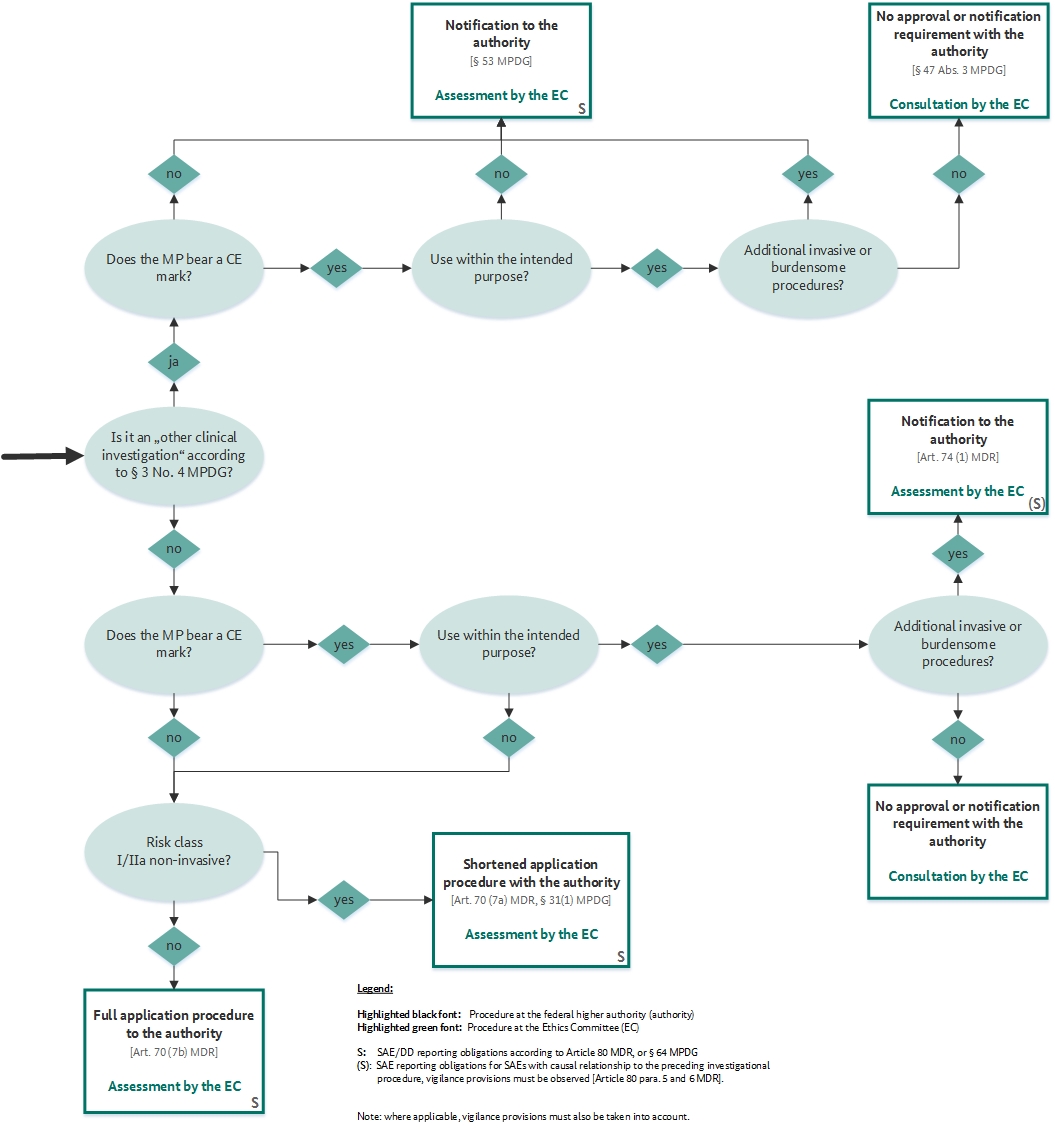
Medical Device Registration under UK MHRA: UKCA Marking Requirements, UK Responsible Person, and More – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

UK Medicines and Medical Devices Act 2021 Includes First UK Legislation to Combat Forced Organ Harvesting - The International Coalition to End Transplant Abuse in China

Centre forms committee for framing new law for medicines, cosmetics, medical devices - Health News | The Financial Express

Medicines and Medical Devices Act 2021: chapter 3, explanatory notes : Great Britain: Amazon.es: Libros

Med Tech Regulatory Reform: The first steps towards a new framework for medical devices in the UK - MedRegs

The combination of medical devices and medicinal products revisited from the new European legal framework - ScienceDirect