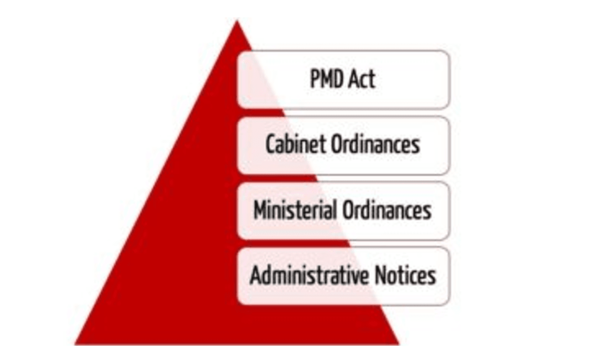
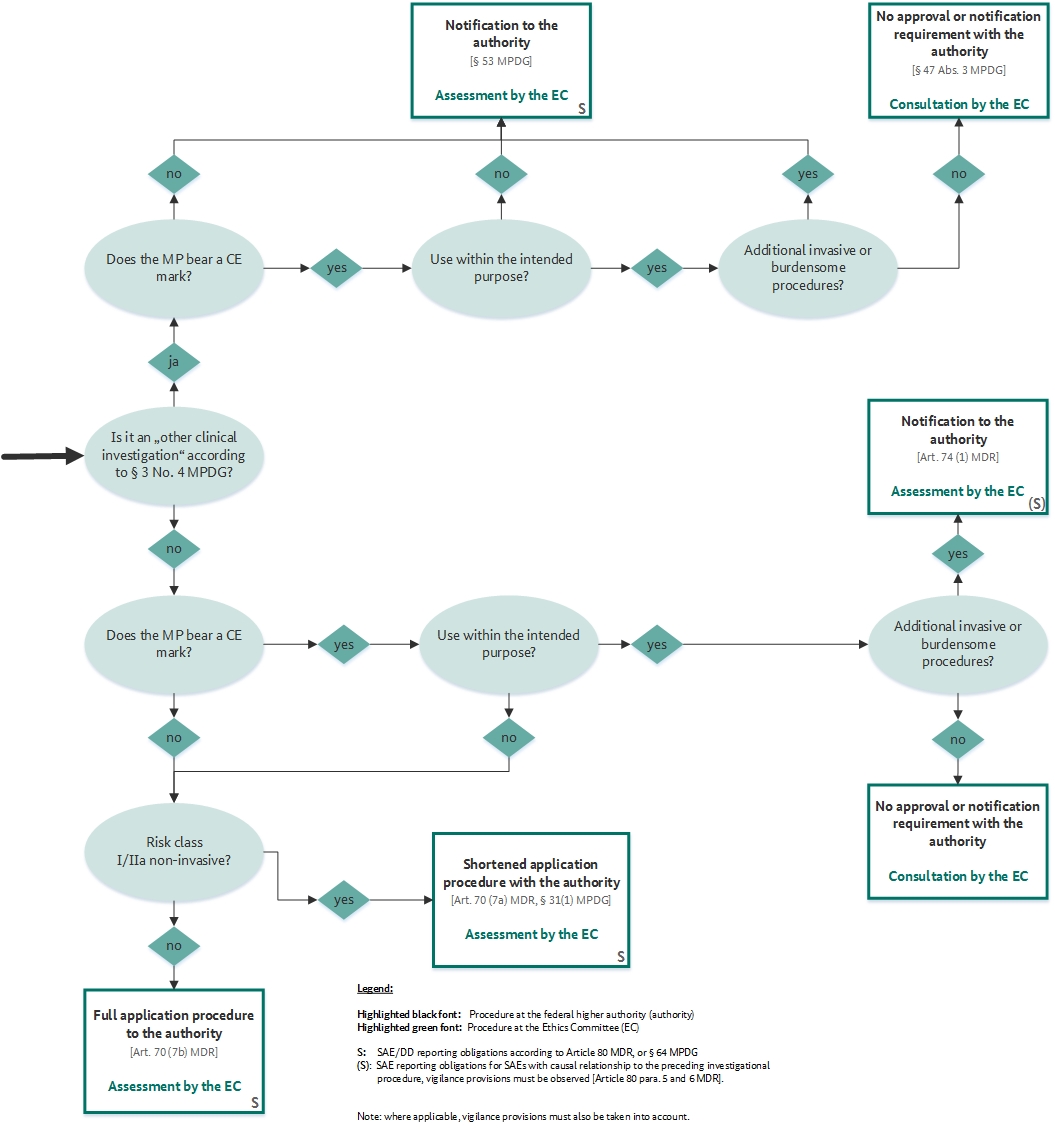
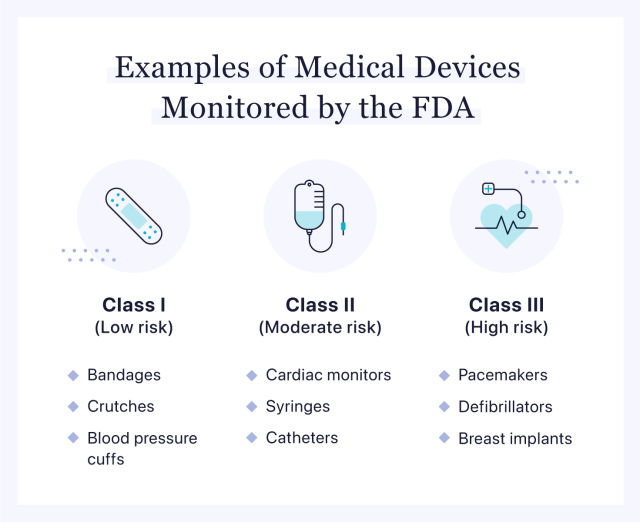
The combination of medical devices and medicinal products revisited from the new European legal framework - ScienceDirect

UK Medicines and Medical Devices Act 2021 Includes First UK Legislation to Combat Forced Organ Harvesting - The International Coalition to End Transplant Abuse in China

New law to regulate drugs, medical devices in age of online pharmacies | India News - Times of India
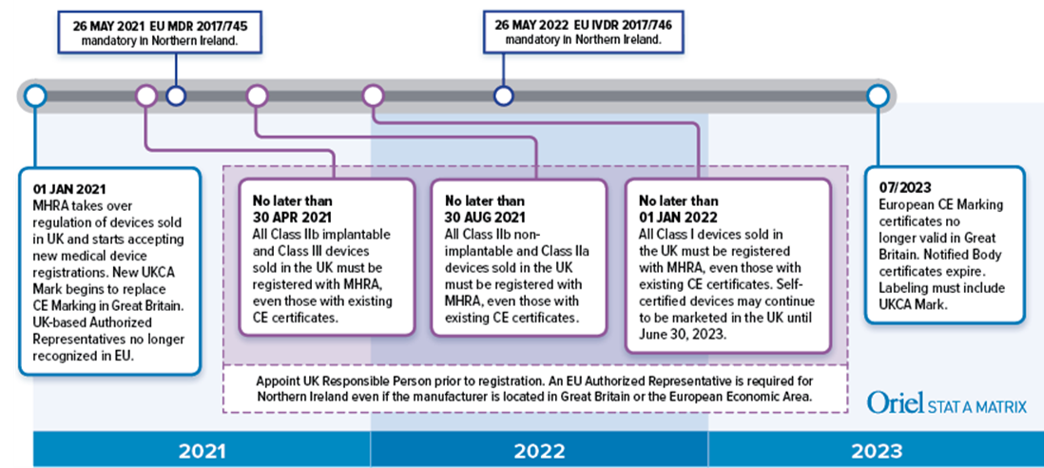
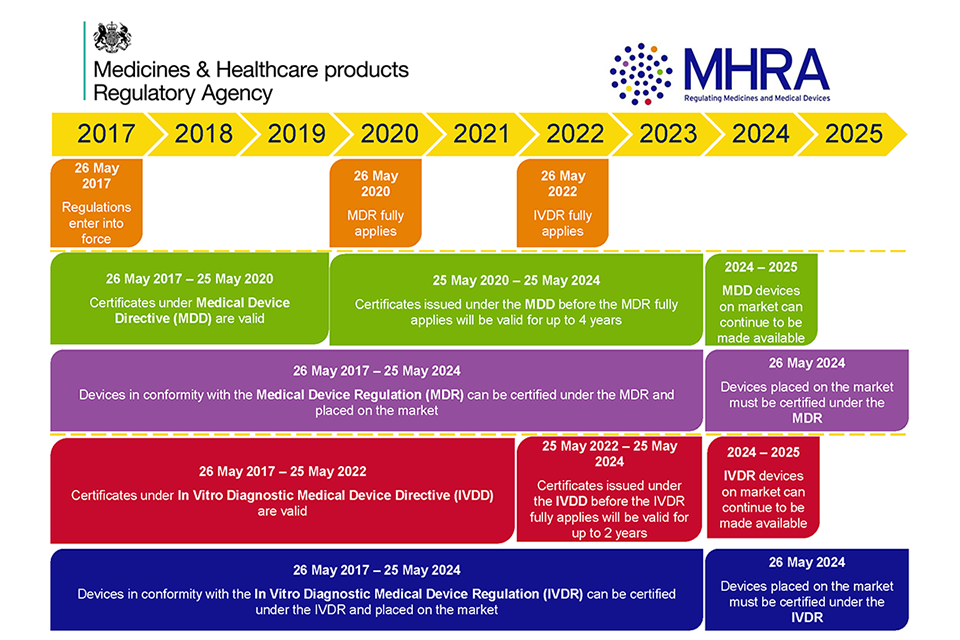
Medical Device Registration under UK MHRA: UKCA Marking Requirements, UK Responsible Person, and More – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
![Withdrawn] Contingency legislation covering regulation of medicines and medical devices in a no deal scenario - GOV.UK Withdrawn] Contingency legislation covering regulation of medicines and medical devices in a no deal scenario - GOV.UK](https://assets.publishing.service.gov.uk/government/uploads/system/uploads/image_data/file/83579/s960_Digital_logo-for-gov-uk.png)
Withdrawn] Contingency legislation covering regulation of medicines and medical devices in a no deal scenario - GOV.UK