A 2000 to 2020 Practitioner's Guide to Chiral Amine‐Based Enantioselective Aldol Reactions: Ketone Substrates, Best Methods, in Water Reaction Environments, and Defining Nuances. - Nugent - 2022 - European Journal of Organic Chemistry - Wiley Online ...
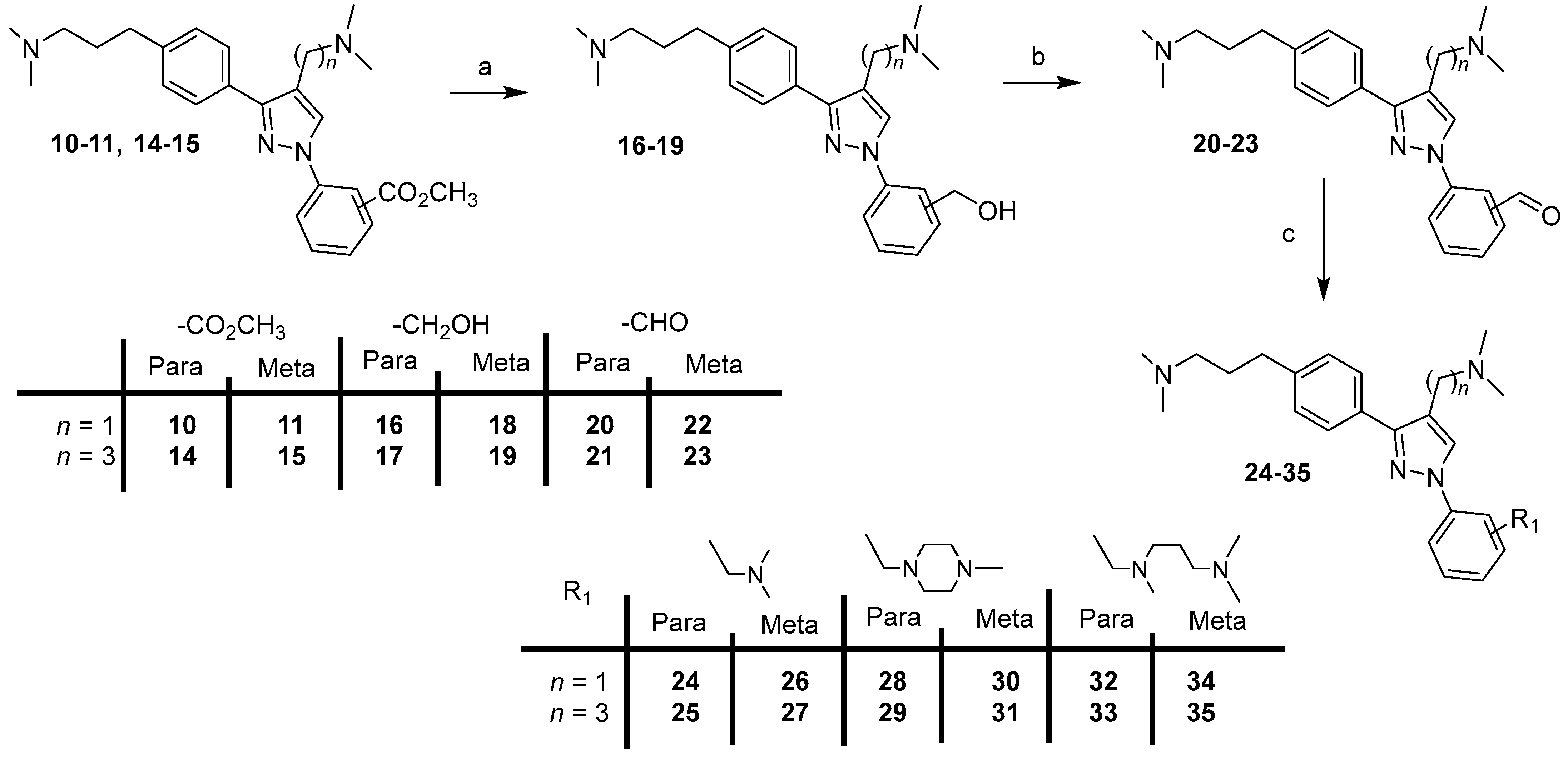
IJMS | Free Full-Text | Discovery of Compounds That Selectively Repress the Amyloidogenic Processing of the Amyloid Precursor Protein: Design, Synthesis and Pharmacological Evaluation of Diphenylpyrazoles

Combinatorial Synthetic Design. Solution and Polymer-Supported Synthesis of Heterocycles via Intramolecular Aza Diels−Alder and Imino Alcohol Cyclizations | ACS Combinatorial Science
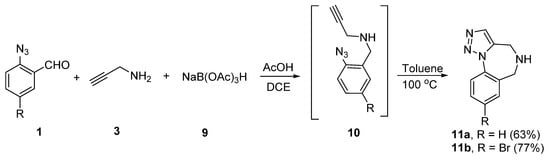
2-Oxindole and related heterocycles: synthetic methodologies for their natural products and related derivatives
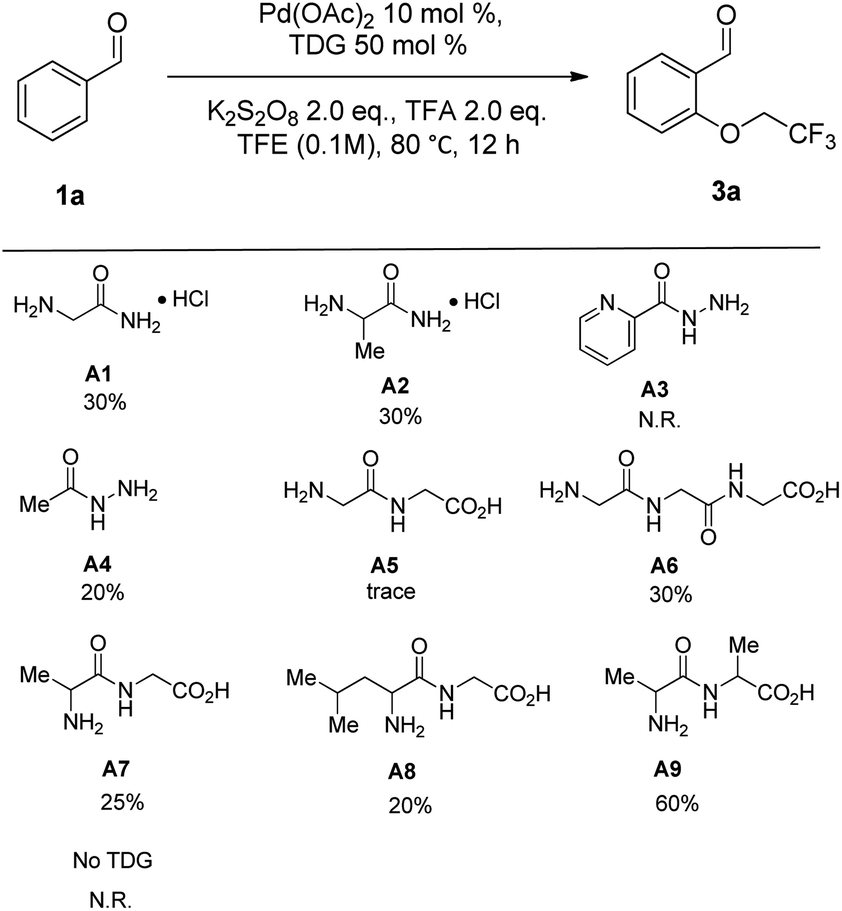
Transient directing group enabled Pd-catalyzed C–H oxygenation of benzaldehydes and benzylic amines - RSC Advances (RSC Publishing) DOI:10.1039/D2RA00241H

Fast-Tracking the l-Lactide Polymerization Activity of Group 4 Metal Complexes of Amine Tris(phenolate) Ligands | ACS Catalysis
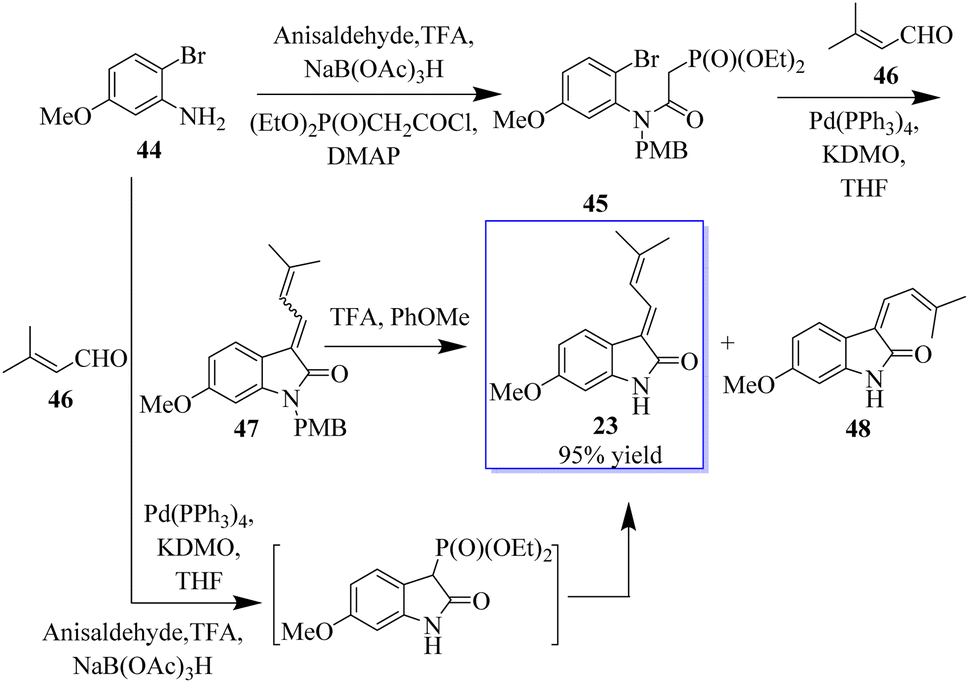
2-Oxindole and related heterocycles: synthetic methodologies for their natural products and related derivatives - RSC Advances (RSC Publishing) DOI:10.1039/D3RA02217J

A Catalyzed and Highly Selective Ester Reduction in the Synthesis of an N-Acylpyrrolidine: Safe Design through Reaction Calorimetry and Modeling | Organic Process Research & Development

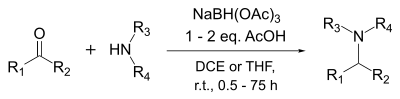
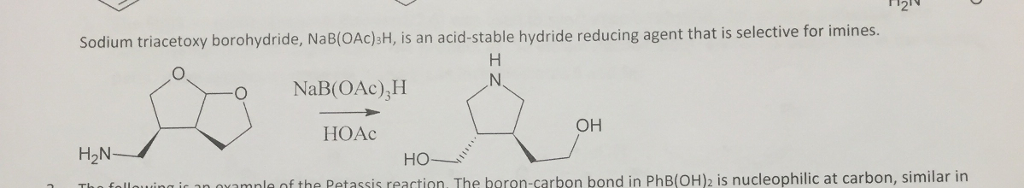


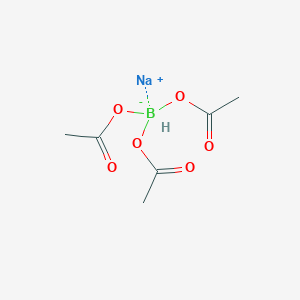

![Reductive Amination - Sodium triacetoxyborohydride [NaBH(OAc)3] Reductive Amination - Sodium triacetoxyborohydride [NaBH(OAc)3]](https://commonorganicchemistry.com/Rxn_Pages/Reductive_Amination/Reductive_Amination_Rxn_6.png)